
Clinical Trials
Search for Clinical Trials at the Case Comprehensive Cancer Center.
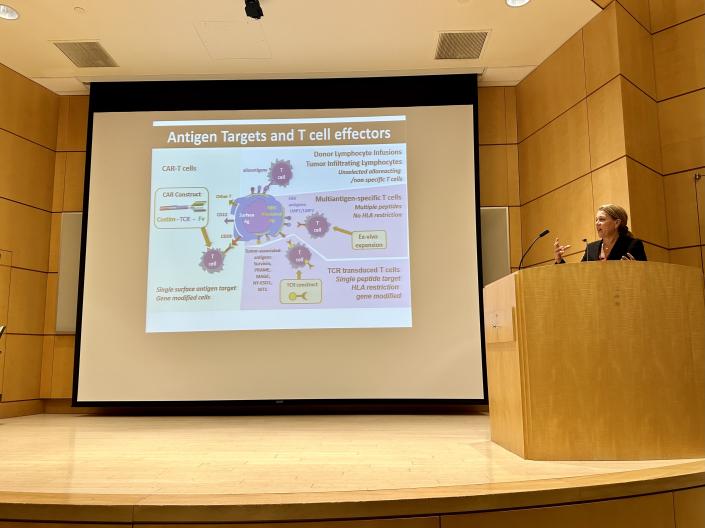
Seminar Series
Join us every Friday for seminars that address topics in cancer research.
Exner, Basilion Receive $2.4M to Continue Work on First Prostate Cancer Cell-Targeted Ultrasound Contrast Agent
March 1, 2024
2nd Annual Microbiome Workshop Gets High Praise
February 9, 2024
Wang Lands NIH Grant for Cancer Biology Research
January 19, 2024
Lathia Lab Research Featured in Nature Cancer 2023 in Review
January 5, 2024




